
Developing human monoclonal antibodies targeting Acinetobacter baumannii
Project title: Lead optimisation of mAbs targeting Acinetobacter baumannii iron acquisition proteins
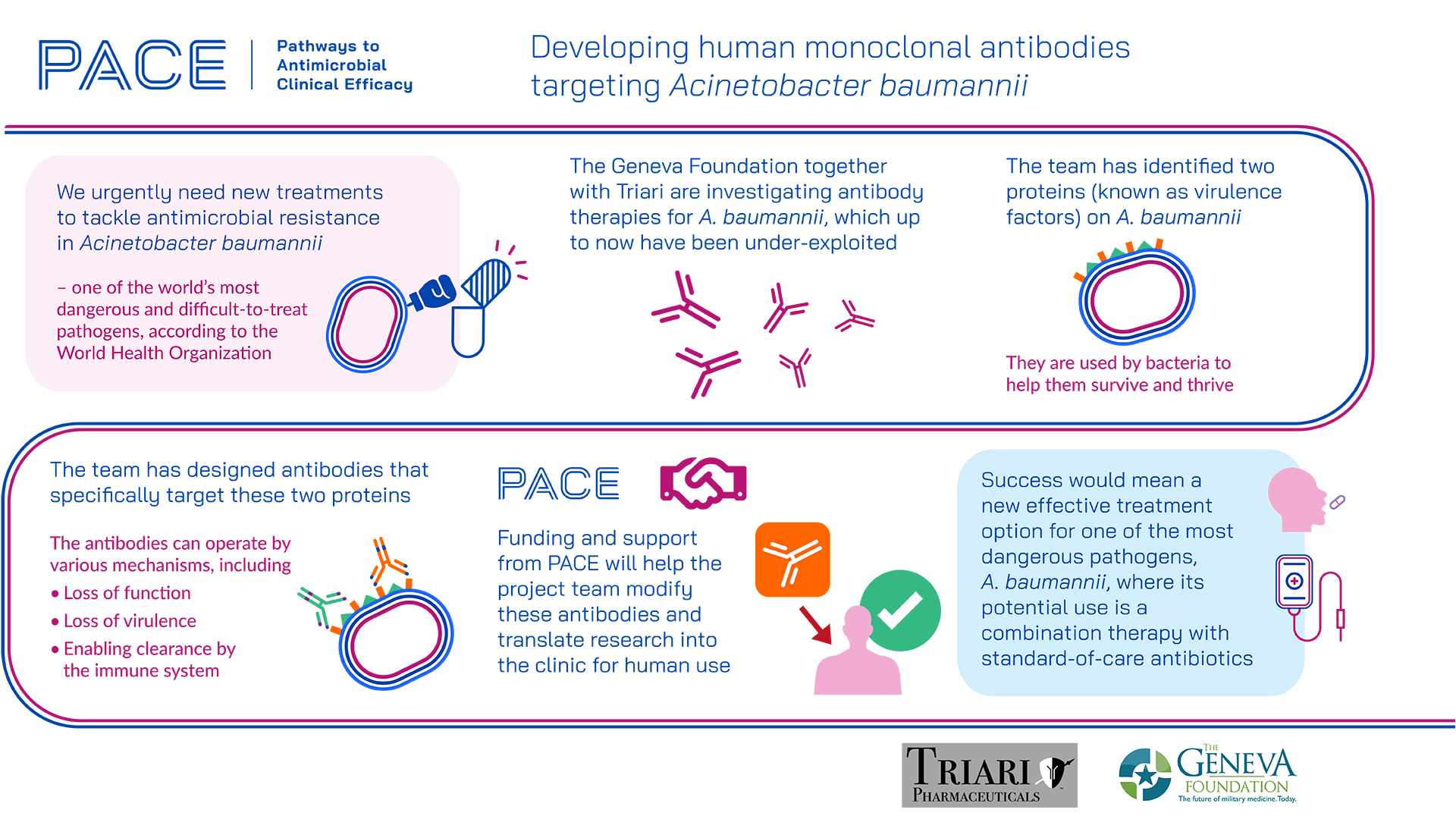
When the Gram-negative bacterium Acinetobacter baumannii is encountered in clinical settings such as acute pneumonia, there are often poor patient outcomes, primarily because some strains are now resistant to all approved antibiotics. The Geneva Foundation, together with Triari Pharmaceuticals, Inc. is addressing this issue by exploring the use of monoclonal antibodies to bind to protein targets on the bacterium’s surface – an approach that leads to loss of function and/or virulence, and ultimately, clearance by the immune system. This antibody approach is well-developed for other conditions such as cancer and viral infections, but up to now, it has remained under-exploited for bacterial infections.
Previous work has identified two target proteins on the bacterium surface, BauA and OmpW2, which are consistently present in most strains because they play essential roles in keeping the bacteria alive via iron acquisition. The team has generated mouse monoclonal antibodies against these targets, which were 70–100% effective in preventing unfavourable outcomes in physiological models of infection. The next step is to modify these antibodies so that the human immune system will accept them, and potentially to improve efficacy. To date, four modified antibodies have been investigated, and the funding and support from PACE will enable these to be developed into leads, to be followed by validation and efficacy testing in vitro and in vivo.
If this approach is effective, it could result in a new treatment to prevent loss of life from Acinetobacter baumannii infection. Importantly, it would also offer several advantages inherent to antibody therapies, including high specificity for the target bacterium (leading to a good safety profile), and the ability to be used alongside small-molecule antibiotics (for greater efficacy at lower doses).
The Geneva Foundation Summary
The Geneva Foundation is a 501(c)3 non-profit organisation dedicated to advancing military and civilian medical research. With over 30 years of expertise, Geneva manages more than 300 research programs across 55 military treatment facilities and federal laboratories worldwide. The foundation specialises in federally funded research that addresses the needs of the Department of Defence (DoD), particularly in infectious disease, biotechnology, trauma care, human performance, and rehabilitative medicine. Geneva’s work directly supports the health, readiness, and operational capabilities of service members, as well as the broader global community. This effort is driven by fostering multidisciplinary collaborations, tailored program management, and facilitating pathways to commercialisation. Additional services include proposal development, financial and contract administration, and award management, all aimed at ensuring successful research outcomes. As a strategic partner, Geneva plays a key role in sustaining and advancing military medicine and supporting the innovation necessary for mission success.
Triari Pharmaceuticals Summary
Triari Pharmaceuticals, Inc. is a pre-clinical biopharmaceutical company that develops novel antibiotic therapies for bacterial infections. The company’s pipeline includes novel antibody treatments against multi-drug-resistant (MDR), gram-negative infections. Triari Pharmaceuticals is currently headquartered in Delaware.
