
Developing a novel mode of action, bladder-targeted, small-molecule, for oral therapy of urinary tract infections
Project title: Leucyl-tRNA synthetase inhibitor for oral therapy of urinary tract infections
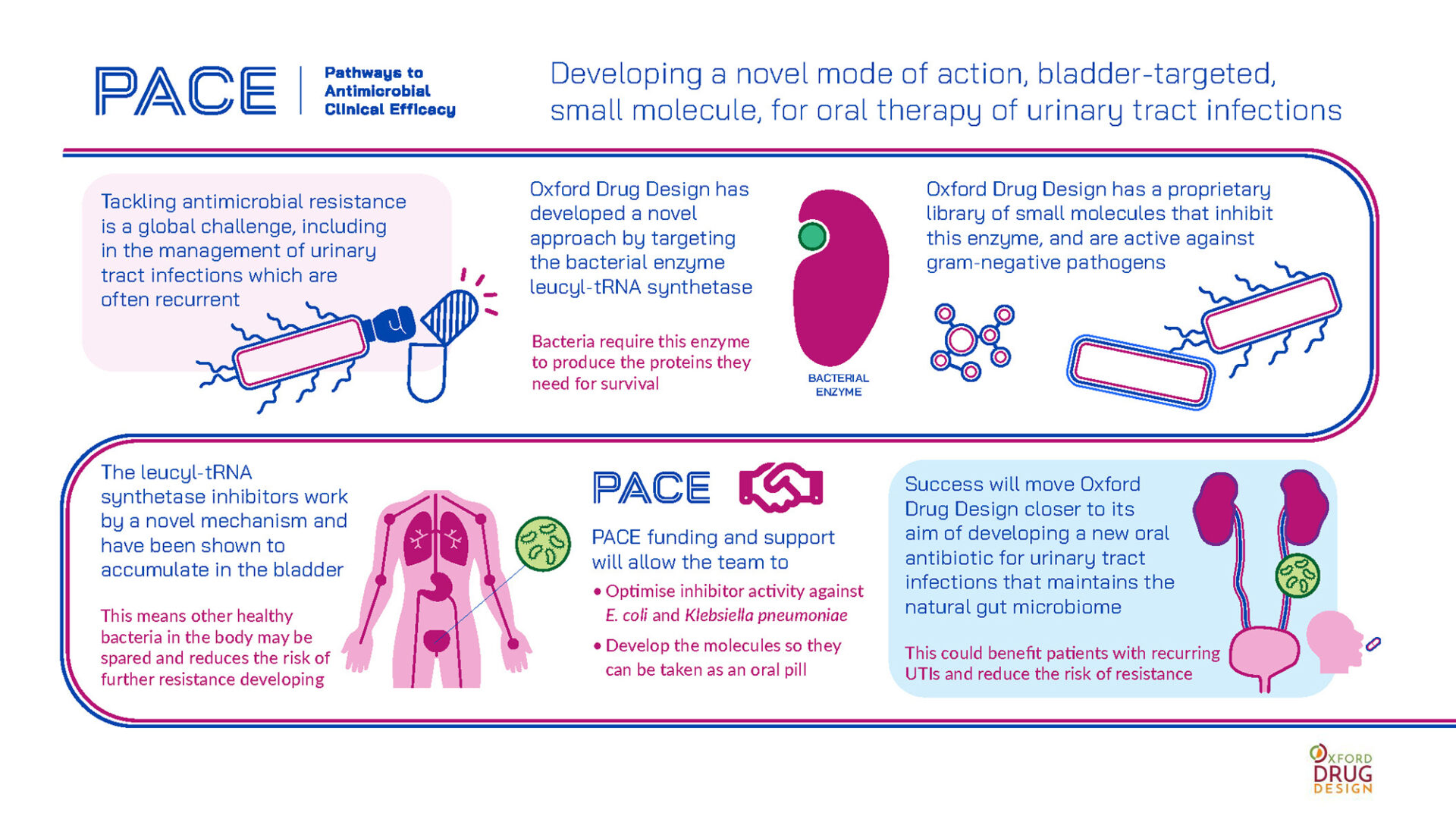
Urinary tract infections (UTIs) are the most common bacterial infection worldwide. By far the most numerous are the so-called ‘uncomplicated’ UTIs, which are nevertheless of concern because of their increasing drug resistance. This is exacerbated by using antibiotics that act through the whole body, with the added problem of collateral damage to the body’s microbiome.
Oxford Drug Design is addressing this challenge by developing a small-molecule oral antibiotic that accumulates in the bladder, where the infection generally resides, and which uses a different mode of action from current UTI therapies. Previous work has identified three potent and selective series of inhibitors of a bacterial leucyl-tRNA synthetase active against several Gram-negative pathogens that frequently occur in UTIs. These inhibitors have activity in physiological models of infection, as well as oral bioavailability.
PACE funding and support will help this work to progress, with key objectives being characterisation of the three inhibitor series, improvement of potency and the pharmacokinetic profile, and evaluation of safety and efficacy. This work will prioritise leads active against E. coli and Klebsiella pneumoniae, including those that produce extended-spectrum β-lactamases and those resistant to carbapenems.
Success in this project will provide an oral antibiotic of a completely novel class and mechanism of action designed with uncomplicated UTIs specifically in mind. Importantly, by being targeted to the bladder, such an antibiotic would reduce the risk of resistance while also minimising damage to the microbiome – which is particularly relevant to patients with recurring UTIs.
Company Summary
Oxford Drug Design has developed a powerful computational platform for computer-aided drug discovery using a unique combination of generative AI, advanced mathematics and novel chemical representations. These groundbreaking methods support its pipeline of internal discovery programmes in therapeutic areas of high unmet medical need.
Two programmes target members of the aminoacyl-tRNA synthetase family of enzymes, of which they have developed a deep understanding and generated a large body of proprietary data, including co-crystal structures as input to the computational methods. Each programme pursues a novel mechanism of action, and for each, it has discovered novel, potent, inhibitor chemotypes and demonstrated in vivo proof of concept.
Oxford Drug Design’s aim is to identify pre-clinical candidates within the next 12-18 months as the next step towards entry into clinical trials.
