2024 Diagnostic Innovation Aims
The goal was to improve the diagnosis and management of bacterial infections with the greatest unmet need and burden of antimicrobial resistance (AMR).
We were looking for projects developing diagnostics to:
- Provide faster results to indicate which antibiotic to use
- Reduce inappropriate antibiotic prescriptions
- Catalyse the move to personalised, narrow-spectrum treatments
Selection Process Overview and Key Metrics
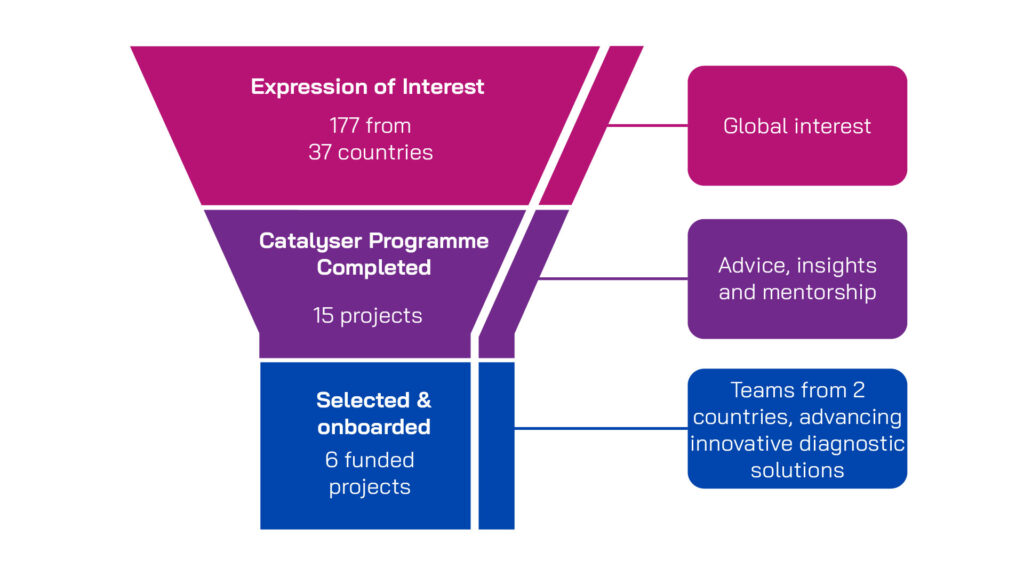
The funding round followed a two-stage application process structured to identify high-potential, strategically aligned projects. Applicants were provided with clear guidance and support throughout.
We received a strong global response to the funding call, with 177 expressions of interest from 37 countries. Each submission was reviewed for its innovative potential against the scope of the funding call. Shortlisted applicants were invited to take part in a structured Catalyser programme – a supportive process designed to help teams refine their project plans and strengthen key aspects of their application.
Fifteen Catalyser participants submitted full applications for funding and support. Final proposals were reviewed by an independent external Scientific Projects Advisory Group for diagnostic innovations and the AMR Community Representatives Committee – a committee of people who have been affected by AMR. Final funding decisions were made by the PACE joint programme management board, taking into account recommendations from the external expert panels, as well as considering strategic and portfolio fit. In total, six projects were selected, onboarded and welcomed into the PACE Portfolio.
Project Onboarding Journey: From EOI to Portfolio
Portfolio outcomes
The 6 funded projects have great potential to improve the diagnosis and management of bacterial infections with high unmet need. Led by innovators from small companies and academic institutions in the UK, in collaboration with partners worldwide, these projects were selected for their novelty, quality and alignment with unmet medical needs and global health priorities.
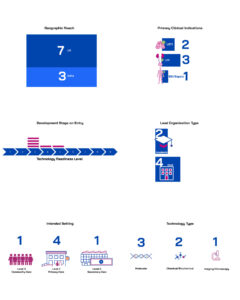
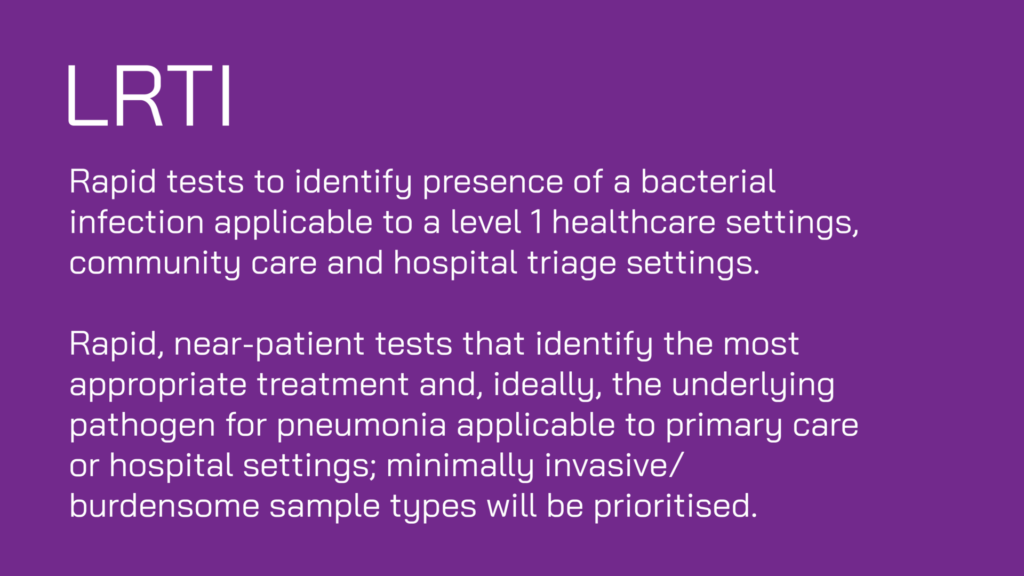
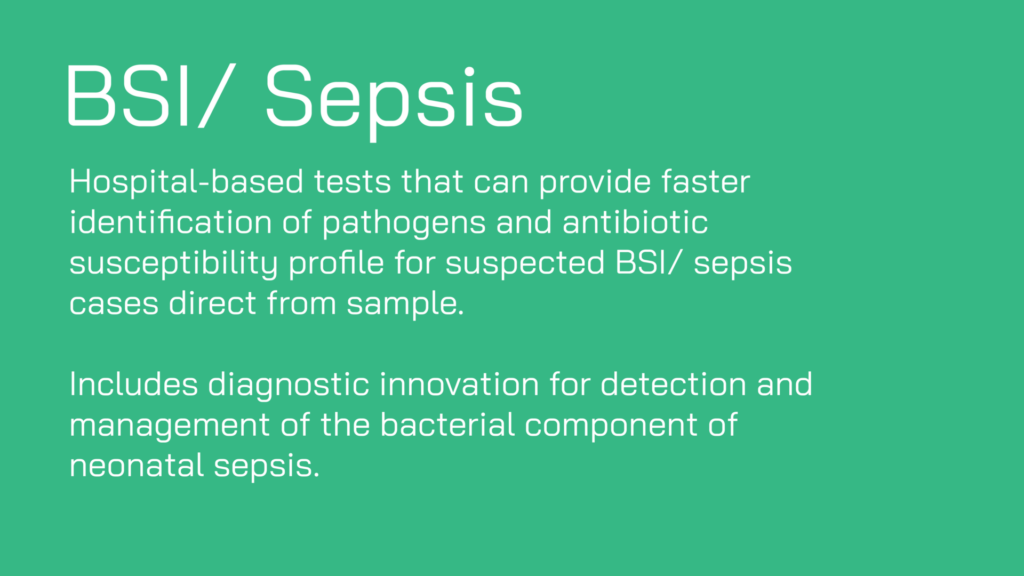
Collectively, these projects cover all three clinical indications in scope for the funding round: lower respiratory tract, urinary tract and blood stream infections/sepsis. Target settings range from Level 0 (community use), through Level 1 (primary care) and Level 2 (secondary care), and are intended for use in low- and middle-income countries (3 projects) and high-income countries (3 projects).
The diagnostic approaches are equally varied, ranging from molecular and biochemical assays to imaging-based technologies. Some aim to provide rapid rule-in/rule-out bacterial detection, either alone (2 projects) or in combination with phenotypic antibiotic susceptibility testing (1 project). The other projects focus on pathogen identification combined with phenotypic antibiotic susceptibility testing (1 project), antibiotic susceptibility prediction using whole genome sequencing (1 project) or through AMR gene marker identification (1 project). The projects entered the portfolio either as technical feasibility studies (Technology Readiness Level (TRL) 3) or product development projects (TRL 4-5).
Together, they form a balanced portfolio of diagnostic tools that strengthen the pipeline of novel solutions aimed at improving patient outcomes and reducing the burden of AMR. To find out more about each project, visit our Portfolio page.