PACE is not just about grant funding; we offer a truly collaborative approach to project development and delivery. Those who secure funding will feel the wider value of a network of potential project partners and receive advice and guidance from some of the best in the field. And we’re there for the long run, so whether you move to the next stage of development or aren’t successful in securing a grant this time around, you can stay plugged into our community and continue to benefit from our expert ecosystem.
Focused on projects with the greatest potential
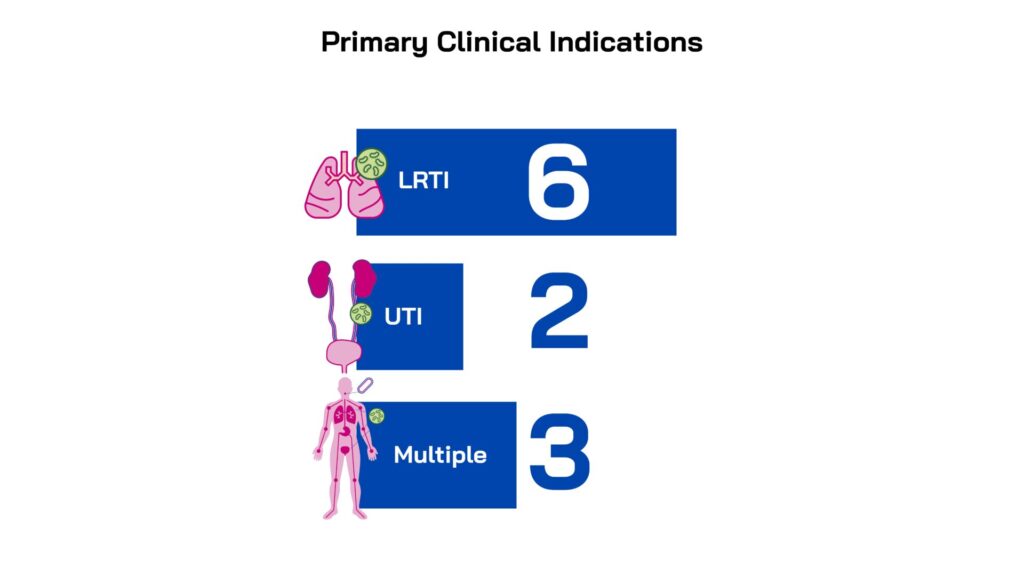
Our 2023 funding call targeted projects directed at the highest-burden drug resistant bacterial infections and indications, as highlighted by the Lancet study and by the WHO priority list. In-scope indications were: lower respiratory tract infections (LRTI); bloodstream infections (BSI); intra-abdominal infections (IAI) and urinary tract infections (UTI). Projects needed to be directed towards one or more of the following drug-resistant bacteria:
Top priority:
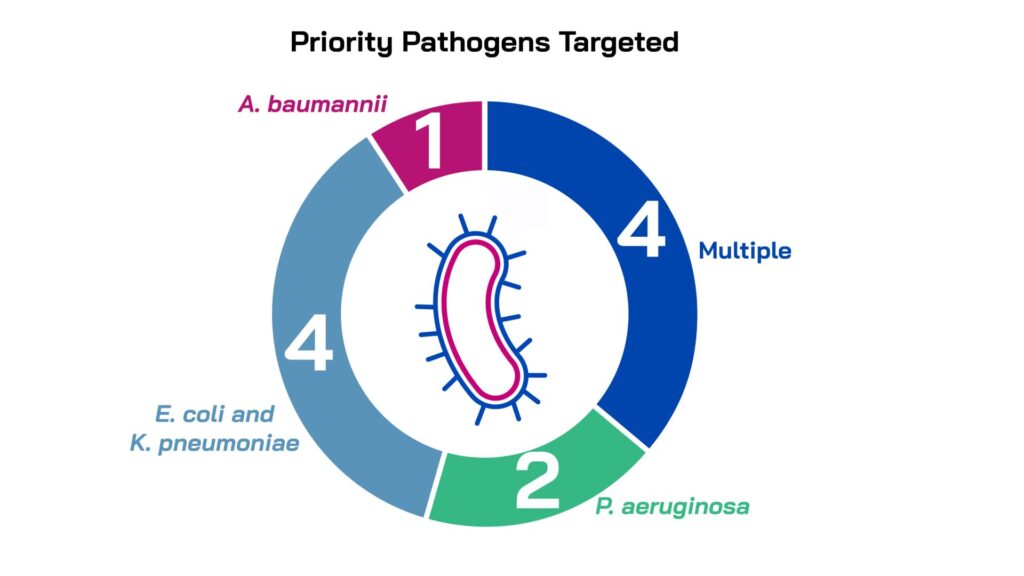
Novel therapeutics applicable for drug-resistant bacterial infections associated with WHO critical priority Gram-negative pathogens Enterobacteriaceae (prioritising E. coli and K. pneumoniae), A. baumannii, and P. aeruginosa
Also eligible:
Novel oral therapeutics applicable to drug-resistant S. aureus
We sought transformational projects, aimed at filling gaps in the clinical pipeline with innovative and differentiated antimicrobials. We encouraged projects that considered how treatments could be tailored to specific patient groups, including the potential use of diagnostics to support targeted use. Our criteria were carefully aligned with those of potential follow-on funders and the NICE subscription model criteria, paving the way from the bench to patient impact. For more details, watch the webinar that accompanied the call launch.
Selection Process Overview and Key Metrics
The funding round followed a two-stage application process structured to identify high-potential, strategically aligned projects and to provide applicants with clear guidance and support throughout.
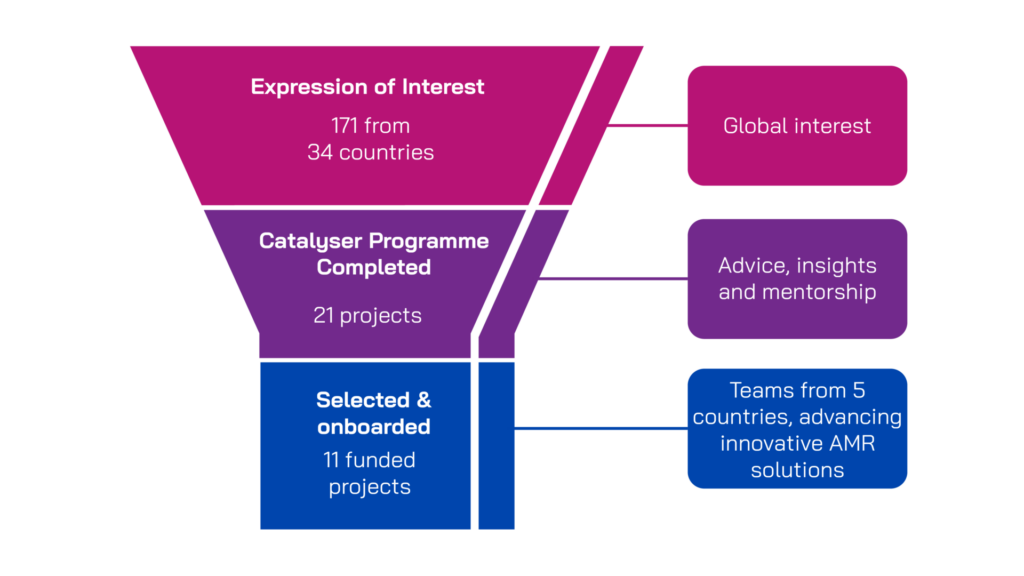
We received a strong global response to the funding call, with 171 expressions of interest from 34 countries. Each submission was reviewed against the scope of the funding call and innovative potential. Shortlisted applicants were invited to take part in a structured Catalyser programme – a supportive process designed to help teams refine their project plans and strengthen key aspects of their application.
Twenty-one Catalyser participants submitted full applications for funding and support from PACE. Final proposals were reviewed by an independent external Scientific Projects Advisory Group for antibacterial therapeutics and a committee of people who have been affected by AMR – the AMR Community Representatives Committee. Final funding decisions were made by the PACE Joint Programme Management Board, which took into account recommendations from the external expert panels, as well as considering strategic and portfolio fit. Eleven projects were selected, onboarded and entered into the PACE Portfolio.
Project Onboarding Journey: From EOI to Portfolio
Portfolio Outcomes
The 11 projects that entered the portfolio from this funding round reflect the diversity and promise of the global innovation landscape. Together, they are making strides towards advancing novel antibacterial therapeutics and strengthening the clinical pipeline with solutions that have real potential to change the trajectory of AMR.
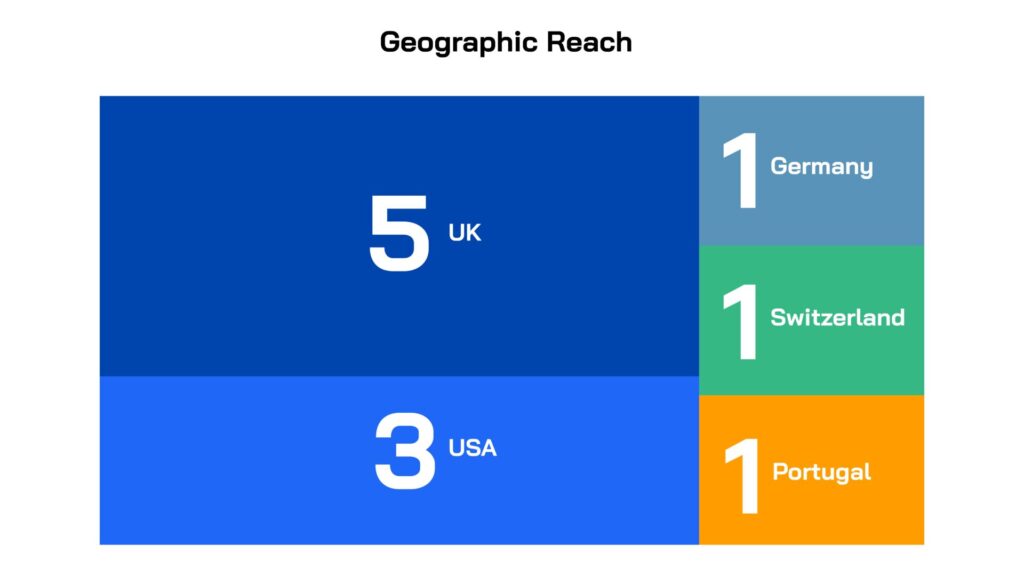
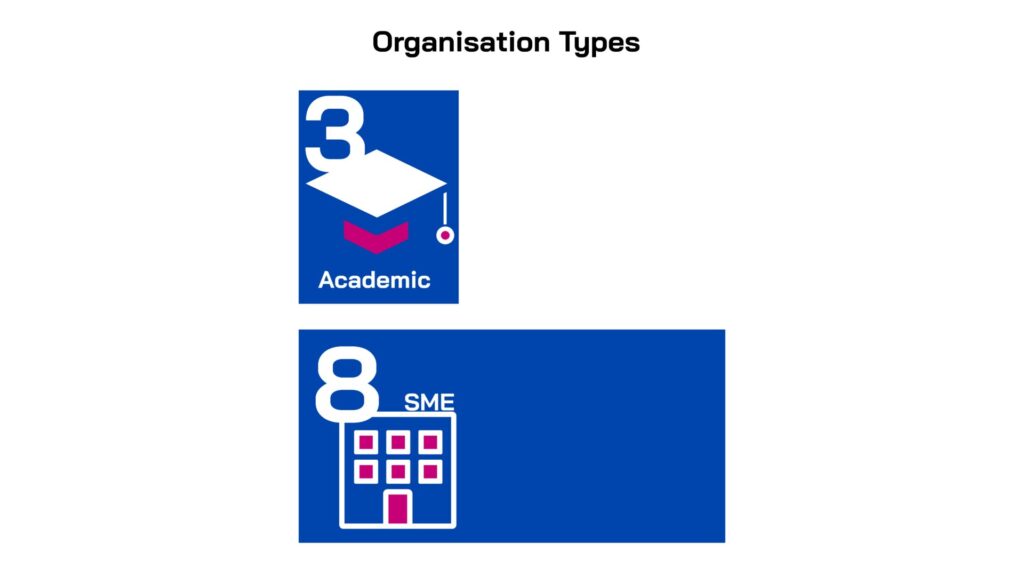
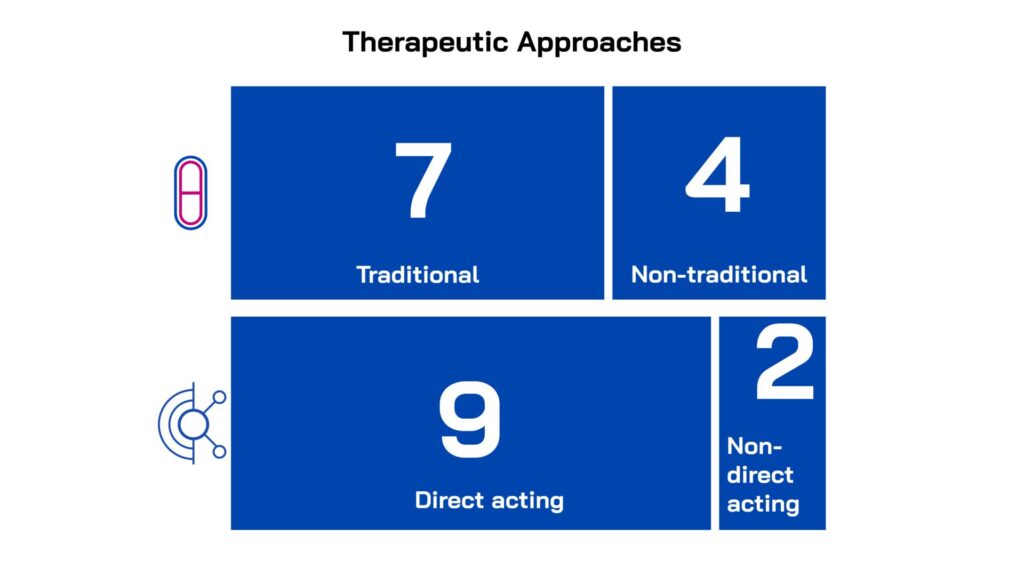
Led by innovators in small companies and academic institutions across five countries, the projects were selected for their novelty, quality and alignment with unmet medical needs. All the projects have the potential to address one or more of the high-burden clinical indications: lower respiratory tract, urinary tract and/or sepsis/bloodstream infections and all target one or more WHO priority gram-negative pathogens. The majority focus on direct-acting therapeutics (9), while two are non-direct acting and are pursuing anti-virulence mechanisms.
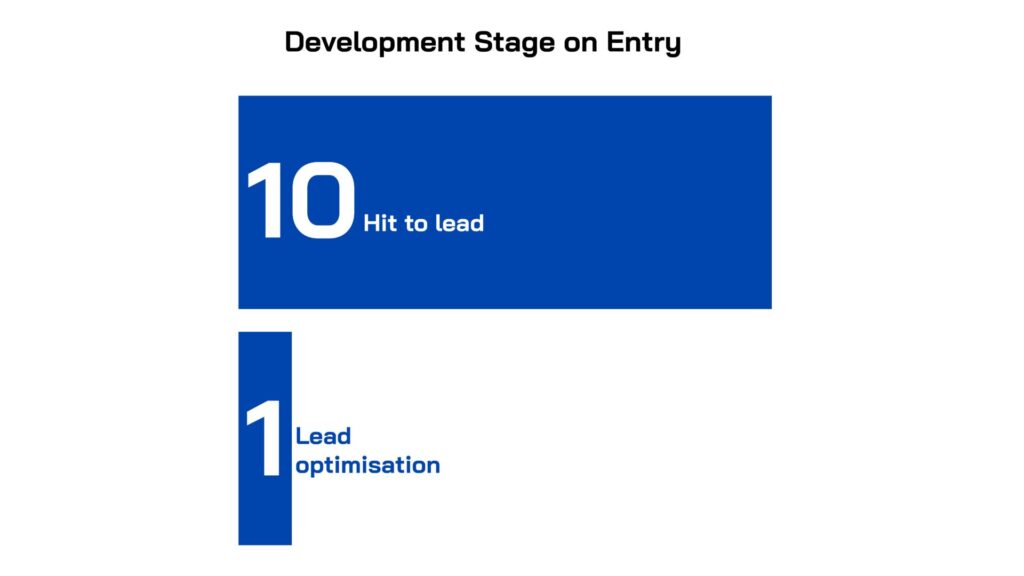
Multiple therapeutic modalities are represented in the portfolio, including traditional small molecules (4) and non-traditional approaches (including antibody (2), protein (1), peptide (1), immuno (1), bacteriophage (1) and peptide-sugar molecule (1) based therapeutic approaches). At the time of portfolio entry, 10 projects were in Hit-to-Lead with one in Lead Optimisation. To find out more about each project, visit our Portfolio page.