2024 Diagnostic Innovations Funding Round (Closed)
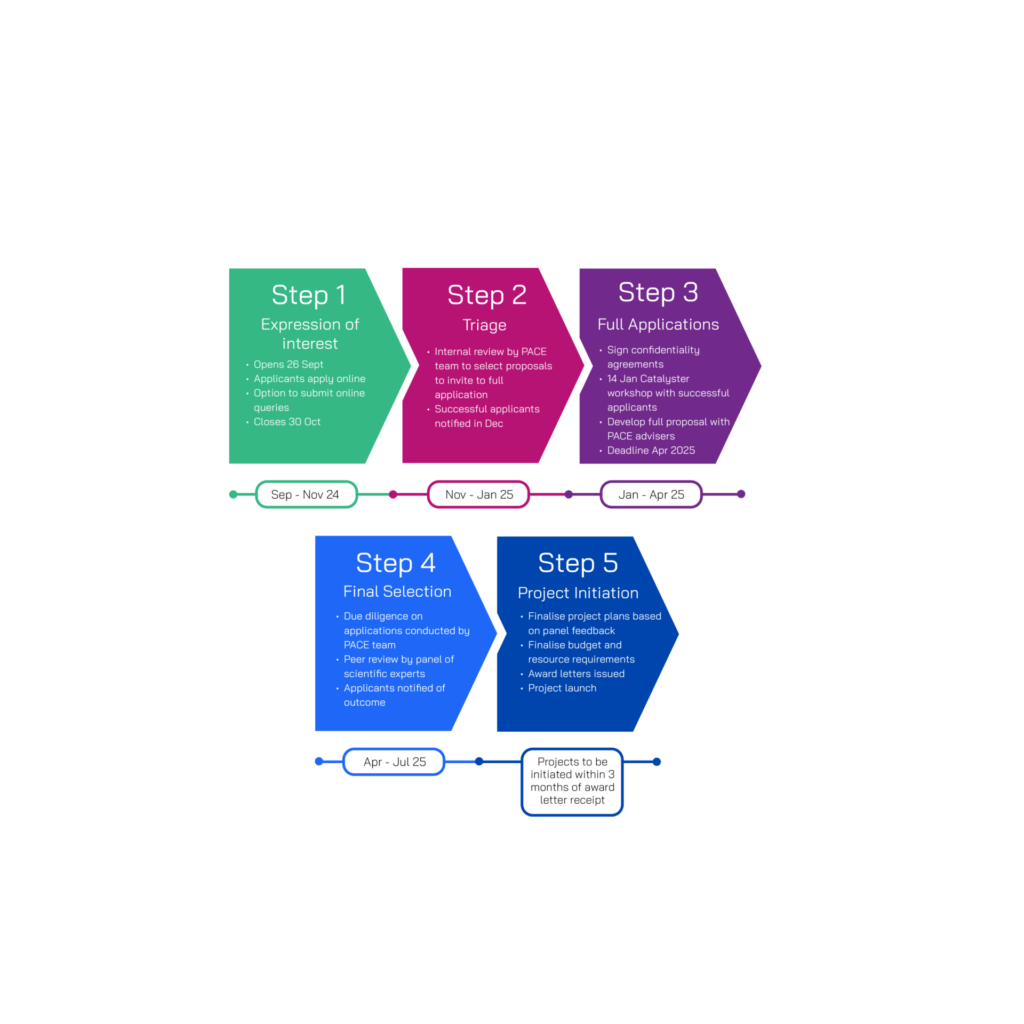
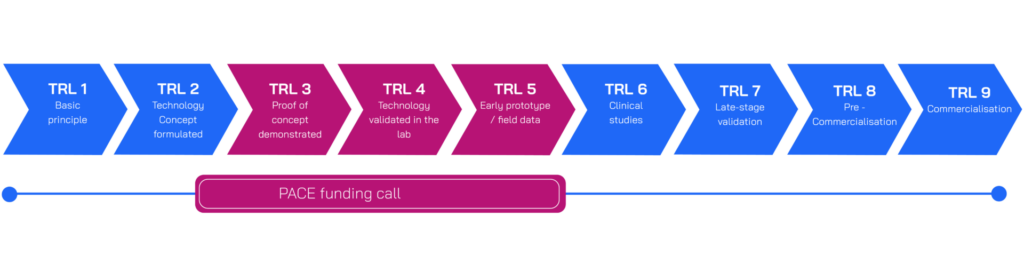
We are looking to drive an exciting and diverse pipeline of early-stage in vitro diagnostics aimed at improving diagnosis of bacterial infections with high unmet need. Up to 8 applicants will be awarded grant funding, with projects expected to last up to two years. There will be awards of up to £300K for technical feasibility projects (Technology Readiness Level (TRL) 3, and up to £1M for product development projects (TRL 4-5). For a visual representation of the TRLs eligible for this funding round, see the below graphic. We held an informational webinar and Q&A on the 2 October. A recording of the webinar and slides are available to view below.
Eligibility
PACE is not just about grant funding; we offer a truly collaborative approach to project development and delivery. Those who secure funding will feel the wider value of a network of potential project partners and receive advice and guidance from some of the best in the field. And we’re there for the long run, so whether you move to the next stage of development or are not successful in securing a grant this time around, you can stay plugged into our community and continue to benefit from our expert ecosystem.
Our funding is open to researches in academia and small to medium enterprises (SMEs) worldwide, including consortia (large diagnostic industry partners permitted, but must cover own costs). Applications should have a single lead applicant, however co-applications are welcome.
What we are looking for (in scope):
- New in-vitro diagnostics in development at TRL stage 3-5 (late TRL 2 considered).
-
- Between Technical Feasibility and Early Prototype including field studies for generating data in relevant environment.
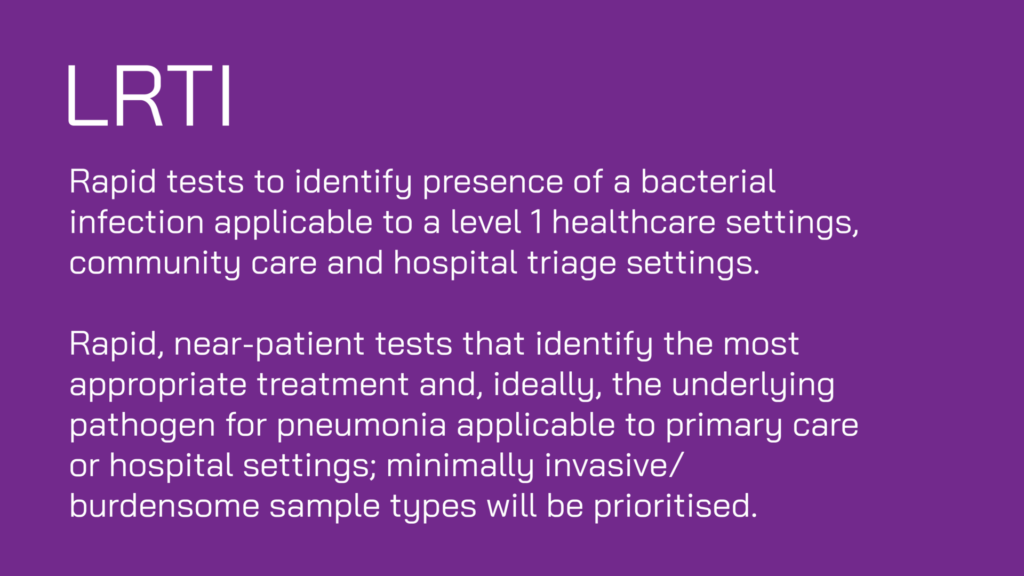
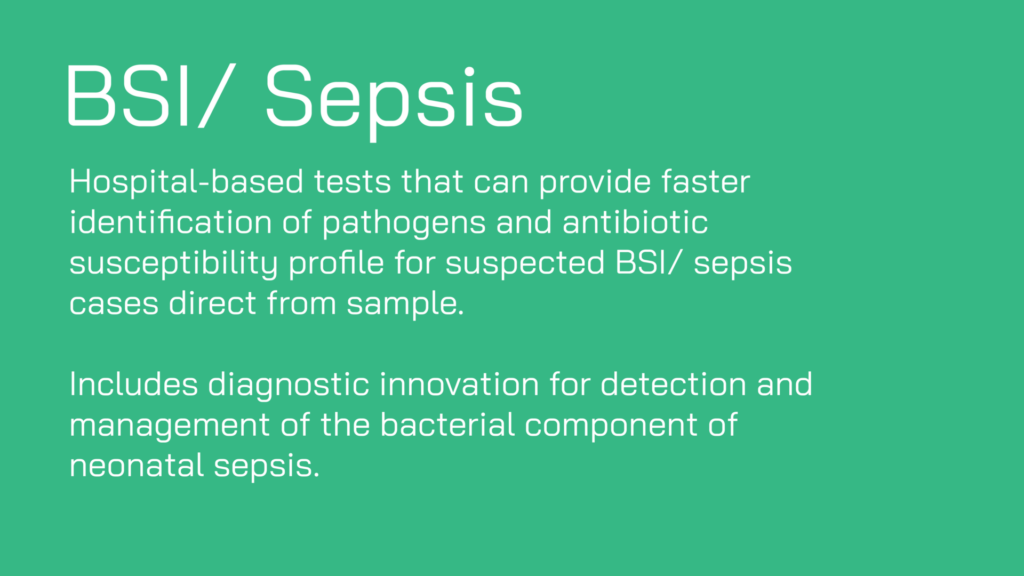
- Adaption of existing diagnostics that could be implemented in new healthcare settings/target regions/countries, or deployed for new indications/use-cases.
- Proposals that include potential for community care (e.g. pharmacy, care home, school, community diagnostic centre) or ‘at-home’ use.
- Analytes can be a direct measurement of bacterial markers or host-based biomarkers, or combination thereof.
- Applications providing solutions tailored to a intended setting. This includes those tailored towards resource-limited settings, including in low and middle income countries (LMICs).
What we are not looking for (out of scope):
- Later-stage diagnostic development (TRL6+), incl.
- Longer term studies in support of regulatory filings such as long-term stability studies.
- Manufacturing of the final instrument/scale-up activities.
- Marketing support including submission of marketing approvals.
- Tests that require central lab infrastructure.
- Standalone: pathogen ID, technology/method/device development, algorithm/ML model development, biomarker identification/validation, pilot lot production of reagents and instruments.
- Surveillance-only approaches and solutions to improve empiric treatment based on surveillance data.
- Diagnostics focused solely on fungal/viral/parasitic infections, sexually-transmitted infections or TB.
- Projects over 2 years in duration.