New call for proposals: 2025 Antibacterial Therapeutics
We are helping to create and advance an exciting and diverse pipeline of therapeutics aimed at treating bacterial infections with high unmet need. Our third funding round focuses on early-stage, novel, antibacterial therapeutics.
- Up to £6 million is available to support up to 8 projects, with a maximum of £1 million available per project.
- Projects are expected to last for up to two years.
- We welcome applications for projects seeking maximum award and for smaller-scale, innovative or higher-risk projects requiring less funding.
- Innovators worldwide are eligible to apply, and there is no requirement for a UK-based partner.

PACE is not just about grant funding; we offer a truly collaborative approach to project development and delivery. Those who secure funding will benefit from a network of potential project partners as well as receiving advice and guidance from some of the best experts in the field. And we’re there for the long run, so whether you move to the next stage of development or are not successful in securing a grant this time around, you can stay plugged into our community and continue to benefit from our expert ecosystem.
Seeking projects with the greatest potential
We are looking for projects, in Hit-to-Lead or Lead Optimisation phases, seeking to treat Gram-negative bacterial infections associated with the highest burden of AMR, as highlighted by the Lancet studies. Indications in scope are: lower respiratory tract infections (LRTI), blood stream infections (BSI) and complicated urinary tract infections (cUTI). Therapeutics must target one or more drug-resistant priority Gram-negative pathogens: Enterobacteriaceae (prioritising E. coli and K. pneumoniae), A. baumannii and/ or P. aeruginosa.
We are seeking the most innovative and differentiated projects with the potential to address drug-resistant infections where treatment options are limited. Funded projects will be expected to demonstrate strong potential to meet the WHO innovation criteria (a new chemical class; a new target; a new mechanism of action; the absence of cross resistance), focusing on candidates with the greatest likelihood of delivering meaningful clinical benefit.
In summary, projects will be prioritised that are developing solutions aligned with the following product profiles:
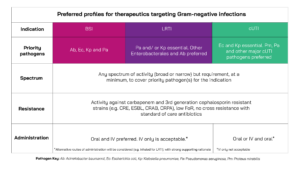
Click image to download.
Eligibility
- Our funding is open to researchers in academia and small and medium enterprises (SMEs) worldwide, including consortia (large pharma partners permitted, but must cover own costs).
- Applications should have a single lead applicant; however, co-applicants are welcome.
- There is no requirement for a UK-based partner.
What is in scope:
- Hit-to-Lead and Lead Optimisation phase projects.
- Any novel targets OR novel chemical classes including but not limited to the following mechanisms; direct-acting and non-direct acting antimicrobials, anti-virulence approaches, immunomodulatory agents, and potentiators.
- Any modality, including but not limited to small molecules, natural products, peptides, nucleic acid-based, antibodies, proteins, complex/targeted modalities, phage; the therapeutic must have an antimicrobial effect.
What is out of scope:
- Target Identification, screening and Hit Identification.
- Projects focussed on late-stage pre-clinical/IND-enabling studies.
- Clinical Phase R&D.
- Antibiotics based on iterations of existing therapeutics e.g. β-lactams.
- Vaccines.
- Interventions focussed exclusively on mycobacteria (including TB), fungal infections, Gram-positive pathogens or Gram-negative pathogens that fall outside the scope of the call’s Target Product Profiles (TPPs).
- Therapeutics solely focussed on out-of-scope indications with no potential to pivot (e.g. topical treatments for skin infections).
- Projects not directly focused on antimicrobial development (e.g. diagnostics, delivery methods, biomarkers, standalone technology or model development).
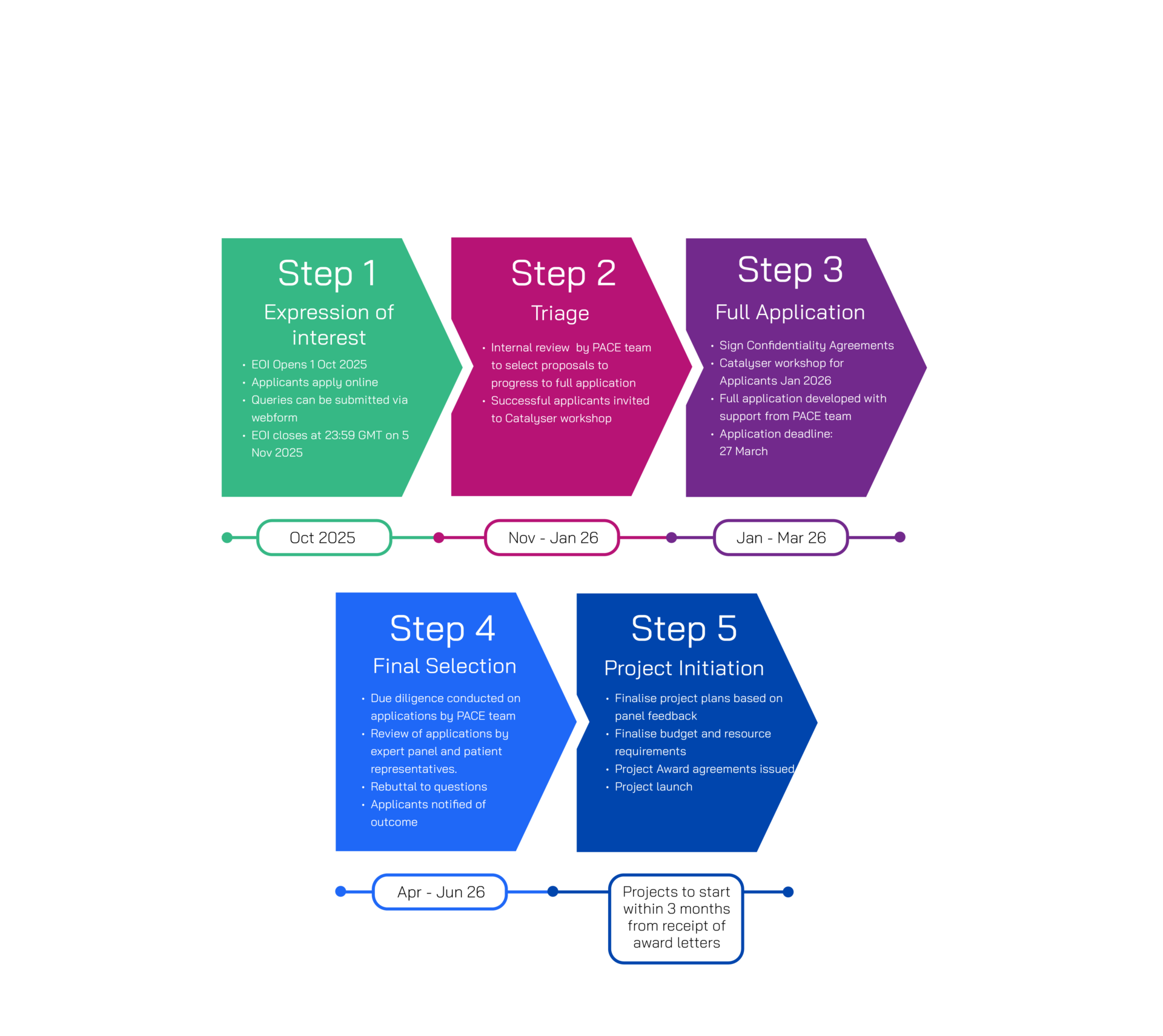
Application Process
Shortlisted applicants will be invited to the full application stage in December 2025.
Catalyser will be available for all shortlisted applicants to support them in developing their full applications, with an initial workshop to take place in January 2026. Final funding decisions will be communicated in July 2026, with an expectation of project initiation within three months of receipt of the award letter. Key steps in the process are summarised.
Please note only applicants that have submitted expressions of interest will be eligible for full applications once selected.

Webinar recorded 8 October 2025
View the slides here:
2024 Diagnostic Innovations Funding Round (Closed)
Our second funding round (launched in September 2024) focused on early-stage diagnostic innovations with the potential to reduce inappropriate antibiotic prescriptions, provide faster results to guide antimicrobial use and clinical decisions and/or catalyse the move to personalised narrow-spectrum treatments.
Learn More

2023 Antibacterials Therapeutics Funding Round (Closed)
Our first funding round (launched in October 2023) focused on early stage, novel antibacterial therapeutics.
Learn More



